Get a quoate
What Are the Benefits of an Air Clean Room? Yield, Safety & GMP Compliance
Published : 2026-01-06In high-end sectors such as precision manufacturing, biopharmaceuticals, healthcare, and food processing, cleanrooms are no longer an optional feature but a standard requirement that determines product quality and corporate survival. Why are companies willing to invest heavily in cleanroom construction? What core competitive advantages do they deliver? This article provides an in-depth analysis from principles to practical applications.

What is a “cleanroom”?
A cleanroom, also known as a dust-free room or clean chamber, refers to a specially designed room where contaminants such as airborne particulates (dust), harmful gases, and bacteria are removed from the air within a defined space. The room maintains controlled parameters including temperature, cleanliness level, internal pressure, airflow velocity and distribution, noise and vibration levels, lighting, and electrostatic control within specified requirements.
- Contamination Control: This is the most fundamental function. Through HEPA/ULPA high-efficiency filters, FFU fan filter units, and specific airflow organization (unidirectional flow or turbulent flow), the concentration of dust particles and microorganisms in the air is controlled within the limits specified by ISO standards (such as ISO Class 5 to Class 8).
- Environmental Stability: A cleanroom is not merely “clean”; it is a constant environment. Through a sophisticated HVAC system, it strictly controls temperature and humidity (e.g., temperature 22±1°C, humidity 50±5%) and pressure differentials (maintaining positive pressure to prevent external contamination ingress), ensuring production processes remain unaffected by environmental fluctuations.
- Structural Integrity: Utilizing dust-free, easy-to-clean specialized color-coated steel panels for walls, anti-static flooring, and rounded corner designs, combined with air lock chambers and air showers, to create a physically isolated controlled environment.
Are cleanrooms just for “looking clean”? Can’t regular air-conditioned rooms suffice?
Absolutely not. Standard air-conditioned rooms merely regulate temperature without controlling microparticles. In the semiconductor industry, a single 0.1-micron dust particle landing on a chip wafer can render the entire chip unusable. In pharmaceutical manufacturing, a single airborne bacterium can contaminate an entire batch of drugs. The core advantage of cleanrooms lies in “invisible control”—the absolute containment of micron-level dust and microorganisms invisible to the naked eye, a feat beyond the capability of ordinary air-conditioned rooms.
Is building a cleanroom a one-time investment, or does it require ongoing, costly maintenance?
Cleanrooms are dynamic controlled environments requiring ongoing maintenance, yet they are not “out of reach.” While initial construction (envelope structures, purification equipment) involves significant investment, subsequent maintenance costs primarily focus on energy consumption and consumable replacements (filters). Through modern modular design and energy-efficient control systems (such as variable-frequency fans and intelligent sleep modes), operational costs have been substantially reduced. Compared to the high scrap rates and recall risks caused by product contamination, cleanroom maintenance expenses represent a highly cost-effective “quality insurance.”
The Four Core Advantages of Cleanrooms
- Significantly Enhancing Product Yield and Consistency (Yield Improvement): This represents the most direct benefit in manufacturing. In fields such as microelectronics, optical lenses, and precision machinery, cleanrooms eliminate airborne particulate interference with delicate components, directly boosting product yield rates from 70%-80% in ordinary environments to over 99%. This dramatically reduces scrap and waste.
- Ensuring Regulatory Compliance and Market Access: For the pharmaceutical, medical device, food, and cosmetics industries, cleanrooms compliant with GMP (Good Manufacturing Practice) or ISO standards are a mandatory prerequisite for obtaining production licenses. Without compliant cleanrooms, products cannot be launched, and companies cannot enter high-end supply chain systems.
- Safety & Cross-Contamination Prevention: In biosafety laboratories (P2/P3/P4) or chemical laboratories, negative-pressure cleanrooms effectively contain harmful viruses, bacteria, or toxic gases within designated areas. These substances are filtered before discharge, protecting personnel from infection while preventing cross-contamination between different experimental samples.
- Establishing a Premium Brand Image and Customer Trust (Brand Image): Maintaining a first-class clean production environment symbolizes a company’s strength. During factory audits, a high-standard cleanroom instills strong confidence in customers—particularly major international clients—demonstrating the company’s hardware capabilities to produce high-quality, highly stable products.
How to Build and Leverage the Advantages of Cleanrooms?
To truly gain the aforementioned advantages, it is not simply a matter of “building a house,” but rather requires following a scientific implementation process:
Step 1: Requirements Definition and Standard Planning
- Define Objectives: Determine your process’s specific cleanliness requirements (e.g., ISO Class 5 for semiconductor manufacturing or ISO Class 8 for food packaging).
- Set Parameters: Establish requirements for temperature and humidity accuracy, pressure differential gradients, illuminance levels, and electrostatic discharge (ESD) protection.
Step 2: Professional Design and Modular Selection
- Zone Design: Design personnel and material flow pathways based on process flow, incorporating changing rooms, buffer zones, and airlock chambers to adhere to the principle of separating contaminated and clean areas.
- Solution Selection: For enterprises prioritizing flexibility and construction speed, a modular cleanroom solution is recommended. Selecting prefabricated wall panels, ceilings, and FFU units can reduce construction time by over 50%.
Step 3: Precision Construction and Equipment Installation
- Main Structure Assembly: Install seamless cleanroom wall panels, ceilings, and anti-static flooring to ensure structural airtightness.
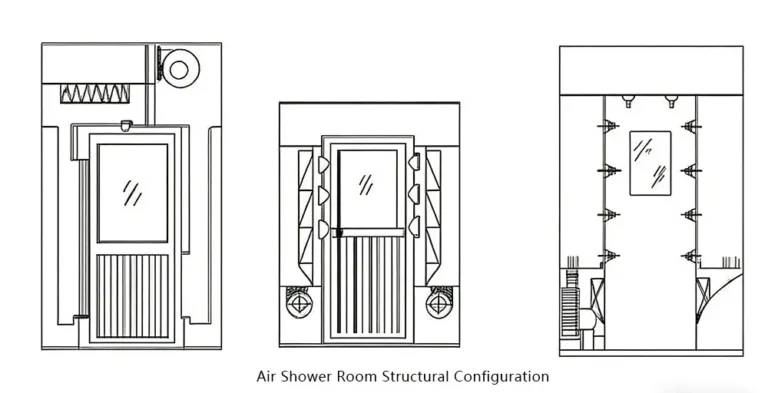
- System Integration: Install high-efficiency particulate air (HEPA) filters, air handling units (AHUs), and air shower systems. The key focus is maintaining cleanliness and sealing of ductwork to prevent secondary contamination.
Step 4: Comprehensive Commissioning and Third-Party Validation (DQ/IQ/OQ/PQ)
- System Balancing: Adjust air volume dampers to ensure pressure differentials in all rooms meet design specifications (e.g., ≥10 Pa between clean and non-clean zones).
- Certification Testing: Engage a third-party agency to conduct particle counting, airborne/settling microbial testing, illuminance measurements, and noise assessments, issuing a valid validation report.
Step 5: Standardized Operations (SOP)
- Personnel Management: Establish strict procedures for changing clothes and hand washing/disinfection. Makeup and jewelry are strictly prohibited inside.
- Maintenance: Regularly monitor differential pressure gauge readings. Promptly replace primary, intermediate, and high-efficiency filters based on alarm prompts.
The Value of Real-World Scenarios
- Result 1: Semiconductor Packaging Plant Achieves Dramatic Yield Improvement A certain optoelectronic chip packaging company previously operated in a standard environment, where dust particle adhesion caused a 15% defect rate of black spots after packaging. After introducing an ISO Class 6 (Class 1,000) modular cleanroom, the number of dust particles in the production environment plummeted. Within the first month of operation, the product yield rate soared to 99.5%, reducing monthly scrap losses by approximately 200,000 yuan. The cleanroom construction costs were recouped in just six months.
- Result 2: Rapid Compliance for Biopharmaceutical Startups A cell therapy startup urgently needed to obtain GMP facility certification within three months to apply for clinical trials. Traditional construction methods for cleanrooms would not meet the deadline. They opted for a modular cleanroom solution, completing the construction and validation of an ISO Class 7 (Class 10,000) core preparation area in just 25 days. The facility successfully passed the on-site inspection by the drug regulatory authority, enabling the company to enter the clinical phase six months ahead of competitors and secure a valuable market advantage.
From rapid delivery to flexible adaptation, from compliance assurance to energy-efficient operations, the advantages of modular cleanrooms are reshaping the global landscape of clean production. Leveraging its core competencies of “factory prefabrication + professional delivery + international compliance,” Boben Modular Cleanroom Manufacturer transforms these advantages into actionable solutions for global clients across electronics, optoelectronics, biopharmaceuticals, precision machinery, and other industries. For European and American enterprises pursuing efficiency, compliance, and sustainable development, choosing Boben Modular Cleanrooms means selecting a cleaner production solution that is more hassle-free, more efficient, and more competitive.