Get a quoate
Class 100,000 Cleanroom Requirements: ISO 8 & GMP Grade D Design Guide
Published : 2025-12-19In industries such as food processing, electronics assembly, packaging, and general medical device manufacturing, a Class 100,000 cleanroom is the most widely used standard for basic environmental control.
Many small-to-medium enterprises (SMEs) face the same dilemma: How do you build a compliant facility without over-designing and wasting capital, or under-designing and risking product contamination or failed audits? Understanding the core requirements—controlling airborne particles, microbes, and environmental parameters—is the key to a successful build.
To help you navigate these requirements, we have prepared the “Class 100,000 Cleanroom Requirements & Free Design Proposal PDF Guide,” featuring parameter tables, design flowcharts, and audit checklists.
Click to contact us and leave your email address. We will promptly send you the free PDF guide and design proposal.
1. Standards Mapping: Class 100,000 vs. ISO 8 vs. Grade D
The term “Class 100,000” originates from the US Fed Std 209E. To ensure compliance in global markets, you must align this with modern international standards:
- ISO 14644-1: Corresponds to ISO 8. This is the standard for most electronics and general manufacturing.
- GMP Standards (EU/WHO): Corresponds to Grade D. This is typically used for auxiliary areas in non-sterile drug production and medical device assembly.
The Goal: To control “dust and bacteria” within specified limits while maintaining stable temperature and pressure. Our PDF guide provides a full cross-reference chart to ensure your design meets local and international regulations.
2. Core Technical Requirements (The Checklist)
A. Airborne Cleanliness & Microbial Limits
This is the primary metric for any cleanroom audit. The environment must be tested “at rest” and “in operation.”
| Parameter | Limit (at rest) | Notes |
| Particle size ≥0.5 microns | ≤ 3,520,000 / m³ | Core focus for electronics. |
| Particle size ≥0.5 microns | ≤ 29,300 / m³ | Core focus for food/packaging. |
| Planktonic Bacteria | ≤ 500 particles/m³ | Applicable to Food & Medical. |
| Settle Plates | ≤ 10/plate | Based on 4-hour exposure. |
B. Environmental Infrastructure: HVAC & Pressure
A cleanroom is only as good as its HVAC system. Class 100,000 environments require specific air change rates to scrub the air effectively.
- Temperature: Typically maintained between 18–26°C (±3°C). Food processing areas may require lower settings (20–24°C), while electronics manufacturing often prefers higher temperatures.
- Relative Humidity (RH): Standardized at 45%–65% ±10%. High humidity risks mold growth; low humidity causes Static Discharge (ESD), which is lethal for electronics.
- Pressure Differential Requirement: Clean areas must maintain a positive pressure differential of ≥5 Pa relative to non-clean areas. This design ensures air flows outward when doors open, preventing contaminant ingress.
C. Ancillary Requirements: Noise, Lighting, and Materials
- Noise Control: Must not exceed 70 dB(A). For precision assembly, we recommend maintaining levels below 65 dB(A).
- Lighting Requirements: Average illuminance must be ≥300 lux. Inspection or packaging areas require ≥500 lux to ensure operational precision.
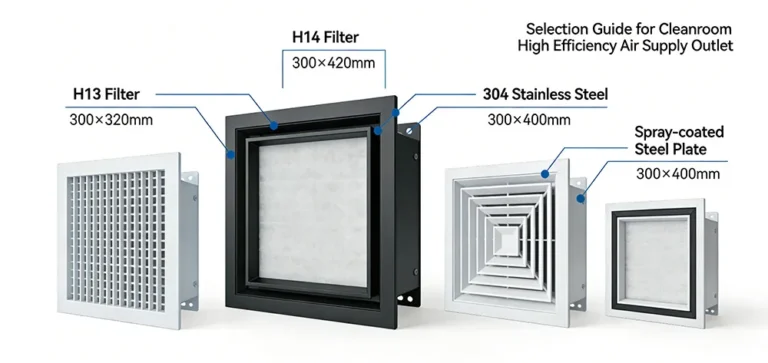
- Surface Materials: Walls and ceilings must use non-shedding, corrosion-resistant materials (e.g., Sandwich Color Steel Panels). Floors are typically Epoxy Resin or PVC. All joints must be coved and sealed to prevent dust accumulation.
3.Industry Applications & Design Pain Points
Class 100,000 cleanrooms are the “entry-level” standard for several booming sectors:
- Food Processing: Bakery production, beverage filling, and specialized packaging.
- Electronics: Circuit board (PCB) insertion, battery component assembly, and plastic casing production.
- Medical & Pharma: Medical dressing production and secondary packaging for pharmaceuticals.
- Printing: High-end aseptic packaging materials.
Common Design Failures:
- Over-Engineering: Spending 40% more than necessary on a system that exceeds your actual industry needs.
- Failed Certification: Improper air-flow design leading to “dead zones” where particles accumulate.
- Maintenance Costs: Designing a system that is cheap to build but consumes massive amounts of electricity.
Our PDF guide analyzes these errors through Real-World Case Studies, helping you avoid the mistakes that cost others thousands of dollars in retrofitting.
How to Download:
Click the comment box on the left
Enter your industry and email address (used solely for sending the guide).
Check your inbox. You will receive the PDF immediately and can opt for a free 1-on-1 consultation with a lead engineer.