Get a quoate
GMP Modular Cleanrooms for Pharma: ISO Compliant & Turnkey Solutions
Published : 2026-01-15In the pharmaceutical and biotechnology sectors, environmental control is not only a guarantee of quality but also a regulatory baseline. As market demands for accelerated new drug development (Time-to-Market) intensify, traditional concrete-built cleanrooms are increasingly struggling to meet requirements due to lengthy construction periods and limited flexibility. Consequently, “pharmaceutical-grade GMP modular cleanrooms” have emerged as the industry’s new favorite solution.
What is “modularization”?
Imagine building with blocks, but these blocks are high-precision industrial prefabricated components. Wall panels, ceilings, doors, windows, and even portions of ductwork are prefabricated in factories, requiring only on-site assembly. This structure typically employs “sandwich” rock wool or honeycomb panels, with surfaces treated for anti-static and corrosion resistance, specifically engineered to withstand rigorous disinfectant wiping.
ISO Compliance and GMP Standards
Pharmaceutical-grade cleanrooms must comply with both ISO 14644 standards (primarily focusing on particle counts, such as ISO 5, 7, 8) and GMP guidelines (e.g., EU GMP Grade A/B/C/D, emphasizing microbial control). This involves more than simply cleaning the room; it requires constructing a sophisticated system encompassing differential pressure control, airflow organization, and temperature/humidity regulation.
The True Meaning of Turnkey?
Many companies wish to avoid the hassle of procuring wall panels, separately hiring HVAC contractors, and then engaging validation agencies. Turnkey solutions mean service providers handle everything from design (DQ), procurement, and construction all the way through validation (IQ/OQ/PQ). In this context, choosing a manufacturer like boben Modular Cleanroom Manufacturers with full-service capabilities significantly shortens the path from blueprint to obtaining production licenses.
When preparing for cleanroom projects, we often hear the following two questions that hit the nail on the head:
Q1: Can modular cleanrooms pass rigorous FDA/NMPA inspections? How airtight are they?
Answer: Absolutely, and in some aspects it even outperforms traditional construction. Modern modular cleanrooms utilize unique aluminum profiles or curved corner connection technology, combined with double-pane insulated glass and airtight doors, to achieve exceptionally high airtightness. For negative pressure isolation wards or high-potency active pharmaceutical ingredient (HPAPI) production facilities, modular structures facilitate tighter gap control, ensuring stable pressure differentials. Provided designs adhere to GMP principles of “separating personnel and material flows” and “preventing cross-contamination,” modular construction is currently the preferred solution for capacity expansion among international pharmaceutical giants (Big Pharma).
Q2: Is a turnkey solution more expensive than subcontracting it yourself?
Answer: At first glance, the total price of a turnkey solution may seem slightly higher based solely on the material list. However, this is a misconception about “hidden costs.” If you subcontract separately, you must coordinate multiple teams—civil engineering, HVAC, electrical, automation, etc. When interface conflicts arise (e.g., ductwork colliding with ceilings), the losses from rework delays (delaying production) far exceed the price difference. The core value of turnkey solutions lies in single-point accountability and high system integration. Long-term, the total cost of ownership (TCO) is often lower.
The adoption of GMP modular turnkey solutions delivers three core benefits:
- Rapid Delivery, Seizing Market Opportunities Traditional construction is subject to weather constraints and concrete curing periods, often taking over six months. Modular production and on-site foundation work can proceed concurrently, with on-site installation completed in just weeks. For CDMO companies urgently needing to produce clinical samples, time is of the essence.
- Flexibility and Asset Preservation Pharmaceutical processes evolve rapidly. Modular wall panels can be disassembled and reconfigured. Should your process change or your facility require relocation, modular cleanrooms can be dismantled and rebuilt elsewhere like equipment, achieving over 80% asset reuse—a capability traditional construction simply cannot match.
- Predictable Quality Control Since major components are manufactured in the controlled environment of boben Modular Cleanroom Manufacturers’ factory, dust contamination and dimensional errors caused by on-site cutting are eliminated. Prefabricated parts typically maintain tolerances within millimeters, ensuring the final assembly’s flatness and aesthetic appeal. This fully complies with GMP requirements for “no dead corners and easy cleaning.”
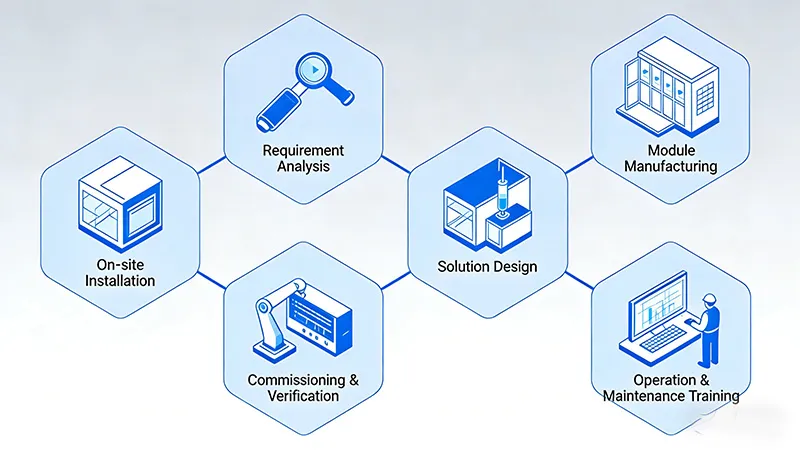
To successfully implement an ISO-compliant cleanroom, it is recommended to follow these rigorous steps:
Step 1: URS Definition and Conceptual Design
Do not start drawing immediately. First, write the URS (User Requirements Specification). Clearly define your cleanliness level (ISO 7 or ISO 8?), temperature and humidity requirements, air changes per hour, and the layout of process equipment. During this phase, determine personnel and material flow paths to avoid cross-contamination risks.
Step 2: Detailed Design and CFD Simulation
Convert the URS into construction drawings. For critical areas, CFD (Computational Fluid Dynamics) airflow simulations are recommended to ensure laminar flow beneath the laminar airflow hood (LAF) meets Class A standards and eliminates vortex dead zones.
Step 3: Factory Prefabrication and Site Preparation
While wall panels, ceiling panels, and return air ducts are being manufactured at the factory, the site will proceed with epoxy floor substrate preparation and the installation of primary air ductwork. Ensure all incoming materials are accompanied by material certificates (e.g., 304 stainless steel certification).
Step 4: Modular Assembly
Install components following the sequence: ceiling first, then walls; interior sections before exterior ones. Pay particular attention to the sealing integrity of the high-efficiency particulate air (HEPA) filter installation, as this is critical for maintaining cleanliness standards. Concurrently, the specialized team from boben Modular Cleanroom Manufacturers will handle complex junctions such as door and window trims, as well as the connection between curved base panels and the floor.
Step 5: HVAC Commissioning and Validation
After system startup, perform terminal air balancing (TAB). The final critical validation phase includes:
- IQ (Installation Qualification): Verify component compliance and secure installation.
- OQ (Operational Qualification): Confirm compliance with pressure differentials, temperature/humidity, and air velocity.
- PQ (Performance Qualification): Simulate production conditions to validate cleanliness maintenance capability.
By implementing the above approach, companies typically achieve significant benefits. Below are several typical outcomes:
- Case Study 1: Dust-Free Renovation of an Aging Factory Building A downtown pharmaceutical plant faced constraints in structural height and load-bearing capacity, precluding major civil engineering modifications. By implementing lightweight modular cleanroom enclosures, the facility successfully established an ISO Class 7 production core within its existing structure without altering the original building framework. This approach resolved the challenge of achieving regulatory compliance upgrades for the aging facility.
- Case Study 2: Significant Energy Savings Thanks to the superior thermal insulation properties of modular panels (a key technological focus for premium manufacturers like boben Modular Cleanroom Manufacturers), combined with intelligent variable-frequency HVAC systems, the delivered workshop achieved approximately 25% lower air conditioning energy consumption compared to traditional workshops of similar scale. This substantially reduced long-term operational costs.
Pharmaceutical-grade GMP modular cleanrooms deliver rapid commissioning and flexible scalability solutions for pharmaceutical manufacturers through ISO compliance assurance and turnkey services. Their core advantages lie in shortening timelines, reducing costs, and ensuring long-term compliance, empowering companies to seize competitive advantages in the market. Choosing modular technology is not only about meeting regulatory requirements but also a strategic investment in agility.




