Get a quoate
GMP Environmental Monitoring Requirements: A Guide for Pharma Cleanrooms
Published : 2026-01-12Core Concepts of Environmental Monitoring in Pharmaceutical Cleanrooms for GMP Compliance
In the pharmaceutical industry, GMP environmental monitoring in cleanrooms is far more than mere “data collection.” It serves as a comprehensive compliance control mechanism throughout the entire production process. Its core function is to ensure pharmaceutical manufacturing remains under constant control through continuous, precise monitoring of critical environmental parameters within cleanrooms. This approach prevents contamination and cross-contamination risks at their source, safeguarding the uniformity and safety of drug quality. This is also one of the core requirements of GMP (Good Manufacturing Practice). Whether it’s China’s GMP, the EU Annex 1, or the FDA cGMP, all impose explicit and stringent regulations on this practice.
From a core composition perspective, GMP environmental monitoring can be broken down into three key dimensions: First, monitoring targets encompass three major categories—“physical parameters + microbial parameters + particle parameters.” Physical parameters include temperature, humidity, pressure differential, air changes per hour, air velocity, etc., which directly impact production process stability. Microbial parameters (settling bacteria, airborne bacteria, surface microorganisms) form the core of contamination prevention and control. Particle parameters (airborne particles) serve as the primary basis for determining cleanliness grades. Second, monitoring states must cover both “static” (equipment operation without personnel activity) and ‘dynamic’ (normal production including personnel operations) scenarios. Dynamic monitoring more closely reflects actual production conditions and is a key focus of GMP inspections. Third, core monitoring principles must adhere to “comprehensive coverage, key emphasis, and data traceability.” All monitoring data requires complete recording and proper storage to ensure traceability for verification. When designing pharmaceutical modular cleanrooms, boben Modular Cleanroom Manufacturers pre-reserve monitoring points and integrate intelligent monitoring interfaces to ensure subsequent monitoring aligns seamlessly with GMP requirements.
Question 1: Are there clear distinctions in the monitoring items and frequencies for GMP environments of different cleanliness grades (Class A/B/C/D)?
Answer: There is a clear distinction. The core principle is that “the higher the grade, the more comprehensive the monitoring items and the higher the frequency.” This is determined by the production purposes of different cleanroom grades. Specific monitoring requirements for each cleanroom grade can be referenced in the table below:
| Cleanliness Level | Core Monitoring Items | Dynamic Monitoring Frequency | Applicable Scenarios |
| Grade A/B | Suspended particles, airborne microorganisms, settled microorganisms, surface microorganisms, temperature and humidity, pressure differential, air velocity | Real-time monitoring, with particulate matter recorded every 30 minutes. | Critical processes such as aseptic filling and freeze-drying |
| Grade C | Suspended particles, airborne microorganisms, settled microorganisms, temperature and humidity, pressure differential | Record once every 1-2 hours | Sterile drug preparation, filtration, and other processes |
| Grade D | Suspended particles, temperature and humidity, pressure differential (microbial can be simplified) | 1-2 times daily | Auxiliary processes such as raw material weighing and outer packaging |
It should be noted that companies must not fall below the minimum GMP requirements, but may appropriately increase monitoring frequency based on their own process risks to develop customized monitoring plans.Click our WhatsApp: +8613862527051 for a free consultation.
Question 2: What should be done when GMP environmental monitoring data exceeds standards? Should production be halted immediately?
Answer: Production does not necessarily need to be halted immediately, but the principle of “immediate response, root cause investigation, and closed-loop rectification” must be strictly followed. Concealing or tampering with data is strictly prohibited. The correct procedure is as follows: Step 1: First, verify whether the monitoring equipment is functioning normally (e.g., calibration status, compliance with sampling methods) to rule out equipment or operational errors. Step 2: If equipment is confirmed functional, immediately isolate the affected area, suspend all related production activities, and expand monitoring coverage (e.g., add surrounding sampling points, shorten monitoring intervals) to trace the pollution source; Step 3: Develop corrective actions based on investigation findings (e.g., enhance cleaning/disinfection protocols, replace high-efficiency filters, optimize personnel operating procedures); Step 4: After rectification, conduct re-monitoring. Production may resume only after confirming compliance with standards. Step 5: Document the entire process comprehensively (including exceedance data, investigation steps, corrective actions, and re-test results). Compile a deviation report and submit it to the quality management department for archiving. boben Modular Cleanroom Manufacturers also assists clients in organizing their exceedance emergency response procedures during after-sales support to mitigate compliance risks.
The Core Benefits of Strictly Implementing GMP Environmental Monitoring
For pharmaceutical companies, implementing GMP environmental monitoring is not an “additional burden,” but rather a key to reducing risks and enhancing competitiveness. First and foremost, it ensures drug quality and safety. Continuous monitoring enables timely detection of environmental anomalies, preventing batch discards caused by drug contamination. For instance, one company once detected a high-efficiency filter leak through suspended particle monitoring, averting the discard of millions of dollars worth of sterile drugs. Second, it facilitates successful GMP inspections. During regulatory audits, the integrity and traceability of environmental monitoring data are critical review points. A standardized monitoring system significantly reduces the risk of failing inspections. Third, it improves production management efficiency. Long-term monitoring data enables trend analysis, helping companies anticipate potential issues (e.g., rising HEPA filter resistance signals timely replacement), thereby preventing unexpected production halts. Finally, it builds market trust. Comprehensive, standardized monitoring records serve as “evidence” of drug quality, enhancing confidence among customers and regulators while laying the groundwork for market expansion.
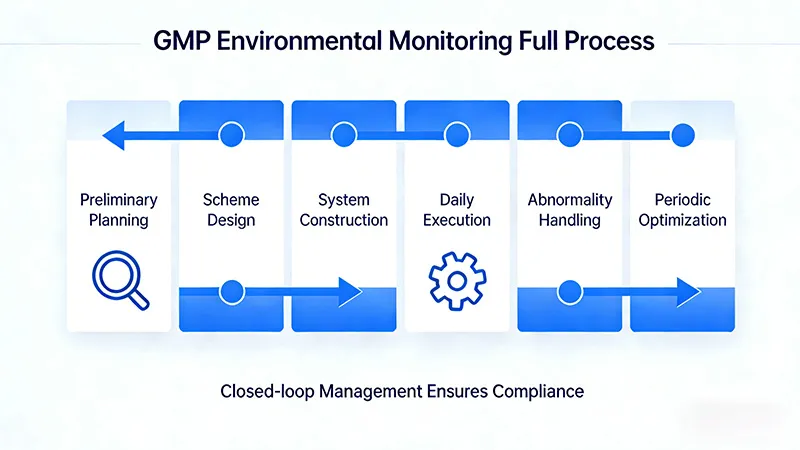
Detailed Steps for GMP Environmental Monitoring
Based on practical experience in the pharmaceutical industry, implementing GMP environmental monitoring requires following a closed-loop process encompassing “planning, setup, execution, and optimization.” The specific steps are as follows:
- Preliminary Planning: First, clearly define the cleanliness levels (Class A/B/C/D) for each clean area and their corresponding production processes. Then, based on GMP regulatory requirements, determine the monitoring parameters, monitoring frequency, and sampling methods for each area. For example, in a Class A filling zone, specify that airborne particle monitoring employs “online real-time monitoring,” with sampling points covering critical areas such as filling ports and material transfer ports. It is recommended to collaborate with specialized manufacturers during planning to avoid omissions in sampling points or unreasonable monitoring frequencies.
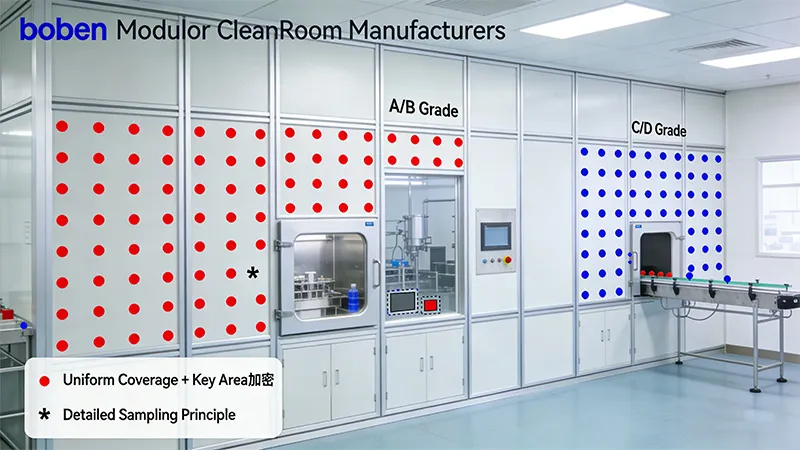
- Monitoring Plan Design: Develop detailed plans based on the layout, focusing on “point placement + equipment selection.” Location layout must adhere to “uniform coverage + key area densification,” with additional sampling points required at critical operational areas (e.g., aseptic workbenches, filling stations). Equipment selection must comply with GMP requirements, utilizing calibrated monitoring devices with valid certificates (e.g., particle counters, air samplers). Equipment must support automatic data logging and export to eliminate manual recording errors.
- Monitoring System Setup: Install monitoring equipment according to the plan, connect it to the data acquisition system, and ensure proper operation. For online monitoring equipment, debug data transmission stability to guarantee real-time data display in the central control room and automatic alarms during anomalies. For offline monitoring equipment, store it according to specifications, calibrate it regularly, and establish equipment usage logs.
- Daily Execution and Documentation: Strictly adhere to monitoring frequencies and sampling methods. For example, sedimentation bacteria monitoring requires the use of 90mm culture dishes, with a placement time of 4 hours and two dishes placed in parallel at each sampling point. All monitoring data must be recorded promptly and accurately, including monitoring time, sampling point, operator, equipment serial number, and data results. Retroactive entries or omissions are strictly prohibited.
- Anomaly Handling and Remediation: Establish an anomaly response mechanism. Upon detecting any exceedance, immediately initiate the aforementioned “root cause analysis-remediation-retesting” process to ensure closed-loop resolution. Concurrently, conduct monthly aggregation and analysis of monitoring data to generate trend reports.
- Regular Validation and Optimization: Conduct monitoring system validation at least once annually to confirm the rationality of monitoring methods and site layouts. Optimize monitoring plans promptly based on process changes, regulatory updates, or trend analysis results. For instance, after introducing new production processes, supplement monitoring sites in corresponding areas.
Case Study on the Implementation of GMP Environmental Monitoring Practices
Case 1: Upgrading the Monitoring System for Class A/B Cleanrooms at a Biopharmaceutical Company
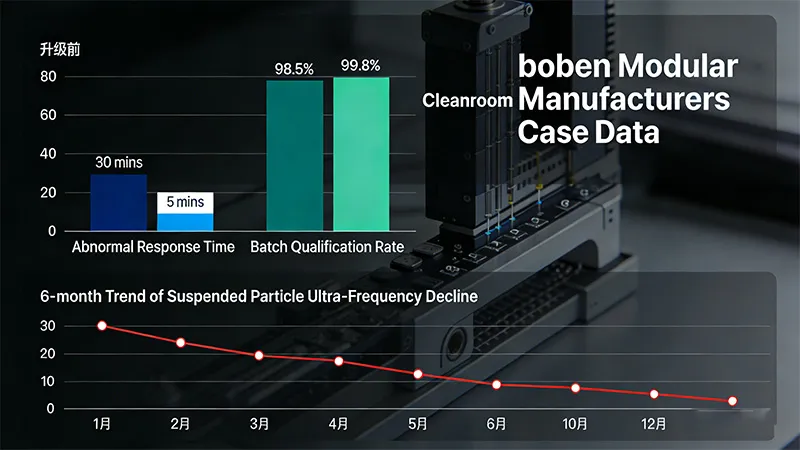
A biopharmaceutical company’s existing Class A/B cleanrooms utilized offline monitoring, resulting in poor data traceability and repeated issues during internal audits. Subsequently, the company implemented boben Modular Cleanroom Manufacturers’ intelligent monitoring solution to establish an online real-time monitoring system. This included optimizing monitoring point layout (adding five critical locations such as filling ports and freeze-dryer discharge ports) while standardizing anomaly handling procedures. Post-upgrade, real-time monitoring of parameters like airborne particles, temperature, and humidity was achieved. The response time for anomaly alerts decreased from 30 minutes to 5 minutes. During subsequent GMP inspections, the monitoring data system passed the audit on the first attempt. Furthermore, the batch pass rate for sterile pharmaceuticals increased from 98.5% to 99.8%.
Case 2: Monitoring optimization of Class C/D cleanrooms in chemical pharmaceutical enterprises
Due to an unreasonable monitoring frequency, a Class C/D clean room in a chemical pharmaceutical enterprise once faced the potential risk of cross-contamination caused by pressure difference fluctuations. Afterwards, the monitoring plan was re-planned according to GMP requirements, and the monitoring frequency for each area was refined (Class C dynamic monitoring once every hour, Class D twice daily). At the same time, operator training was strengthened, and the sampling process was standardized. After optimization, the rate of pressure difference fluctuation exceeding the standard decreased from 5% to 0.3%, and the risk of cross-contamination was completely eliminated. The trend analysis of monitoring data also revealed the issue of clogged air conditioning system filters, which was rectified in advance to avoid production halts, resulting in an annual reduction of economic losses of approximately 800,000 yuan.
boben Modular Cleanroom Manufacturers delivers comprehensive solutions for pharmaceutical cleanrooms. Its modular cleanroom designs not only meet stringent GMP requirements for cleanliness, temperature/humidity, and pressure differentials, but also enable real-time environmental control through intelligent monitoring systems. For instance, in sterile drug production, boben’s solutions help enterprises avoid common operational errors—such as improper equipment cleaning or unclear material labeling—thereby enhancing production compliance.
Furthermore, boben’s modular design enables rapid deployment and flexible adjustments to accommodate varying production needs. Its optimized airflow organization technology ensures air changes per hour (ACH) and differential pressure management comply with GMP standards, delivering reliable environmental assurance for pharmaceutical manufacturing.




