Get a quoate
How to Ensure GMP Compliance for Modular Cleanrooms? 4 Critical Keys
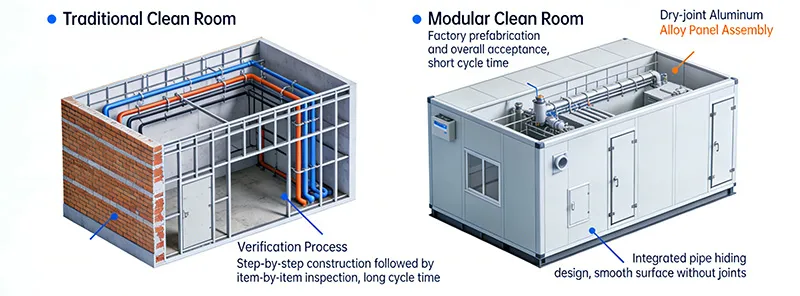
Published : 2026-01-08For pharmaceutical and biotechnology companies, GMP (Good Manufacturing Practice) compliance is not an option—it is the baseline for survival. Traditional cleanroom construction, with its 12-18 month timelines and complex validation processes, often leads to missed time-to-market windows. In contrast, modular cleanrooms are becoming the new standard for compliance due to their rapid deployment and flexible adaptability.
However, to ensure a modular cleanroom successfully passes GMP validation, one must grasp the core logic and critical steps. As an expert deeply rooted in the industry for years, Boben Modular Cleanroom Manufacturers is here to clarify the path with our mature experience.
The Core Logic: Stability & Reliability
First, it is crucial to understand that the core of GMP validation for modular cleanrooms is proving the “stability and reliability of the controlled environment.” Unlike traditional methods, the prefabricated nature of modular cleanrooms paves the way for compliance right from the design phase.
Boben Modular Cleanroom Manufacturers strictly adheres to the latest FDA, EMA, and Chinese GMP standards during the design phase. By utilizing BIM 3D modeling to simulate airflow patterns (CFD) and personnel/material flows in advance, Boben eliminates contamination risks caused by irrational layouts—laying a solid foundation for compliance validation.
| Comparison Dimensions | Traditional Cleanrooms | Modular Cleanrooms | GMP Validation Advantages |
| Validation Cycle | 6–8 months | 2–3 months | Reduced by 40%–60%, enabling rapid production launch |
| Material Compliance | On-site material selection makes certificate traceability difficult | Factory prefabrication ensures complete material documentation | Reduces the risk of non-compliant materials |
| Airflow Organization Validation | On-site adjustments may create dead zones | Factory simulation testing ensures precision and control | Cleanliness compliance rate increased by 30% |
| Rectification Costs | 15%-20% of total investment | 5%-8% of total investment | Significantly reduce verification and rectification losses |
Step 1: Material and Structural Compliance Control
The first step in validating GMP compliance is the strict control of materials and construction. The core GMP requirements for cleanroom materials are “non-shedding, easy to clean, and corrosion-resistant.” Any gaps or porous materials can become hidden hazards for contamination.
Boben selects color steel plates that have undergone specific anti-bacterial treatments. The surfaces are smooth and non-porous, and joints are seamlessly connected using specialized sealing strips to reduce microbial growth at the source. All materials come with compliance certificates to meet the stringent requirements of EU GMP Annex 1, clearing the first hurdle of validation.
Step 2: Implementation of the Full-Lifecycle Validation System
This is the most critical link. Professional modular cleanroom manufacturers complete a significant portion of the validation work in advance, drastically reducing on-site pressure.
Boben’s modular cleanrooms complete the core content of Design Qualification (DQ) and Installation Qualification (IQ)—such as HVAC system airflow testing and HEPA filter integrity testing—during the factory prefabrication stage. Once on-site assembly is complete, the focus shifts to Operational Qualification (OQ) and Performance Qualification (PQ), verifying key metrics like temperature/humidity precision (±1℃), differential pressure stability, and cleanliness levels. As shown in industry data, this approach reduces the overall validation cycle by over 40%, allowing enterprises to reach production faster.
Step 3: Continuous Compliance Assurance During Operations
GMP validation is not a one-time event; regulatory bodies conduct periodic inspections of environmental monitoring data continuity.
Boben equips its cleanrooms with intelligent monitoring systems to track critical parameters such as airborne particles, microorganisms, temperature, and humidity in real-time. Data is automatically stored and traceable, making surprise inspections manageable. Furthermore, the modular design supports flexible expansion. When production processes change, modules can be simply adjusted and re-validated without large-scale demolition, adapting easily to regulatory upgrades.
Why Partner with Boben?
Finally, choosing a reliable manufacturer is the shortcut to compliance validation. Many companies face issues like airflow turbulence and poor sealing due to selecting non-professional vendors, leading to repeated rectifications and increased costs.
Boben’s project team boasts over 13 years of GMP validation experience, providing full-process services from design and production to installation and validation. We have successfully assisted over 500 global pharmaceutical enterprises in passing GMP certification on the first attempt. Our modular solutions shorten construction time by 30-50% and reduce costs by 15-30%.
In summary, the core of GMP validation for modular cleanrooms lies in converting “prefabrication advantages” into “compliance certainty,” forming a closed loop from materials and design to validation and operations.
Boben Modular Cleanroom Manufacturers uses standardized processes and customized solutions to make compliance validation more efficient and worry-free. If your enterprise is planning a cleanroom project and wants to pass GMP validation quickly while controlling costs, contact Boben’s professional team today for a targeted solution.