Get a quoate
What is a Cleanroom in Pharma? Standards, Grades & ISO Compliance
Published : 2026-01-05In the pharmaceutical industry, a cleanroom refers to a specialized space that provides a controlled environment for drug production by regulating environmental parameters such as airborne particulate matter, microorganisms, temperature and humidity, pressure differentials, and airflow organization. Its core objective is to prevent cross-contamination and ensure the sterility and consistency of pharmaceutical products, making it indispensable particularly in the production of sterile preparations such as injectables and ophthalmic formulations.

Cleanliness Classification (China GMP vs. ISO 14644)
| GMP Grade | ISO 14644-1 Equivalent | Maximum Allowable Particle Count ≥0.5μm (Static) | Primary Application |
| Class A | ISO 5 | 3,520 particles/m³ | High-risk operation areas such as aseptic filling, stopper transfer, and sterile connections |
| Grade B | ISO 5 (Background) | 3,520 particles/m³ (Dynamic) | Background environment for Grade A areas; preparation prior to aseptic compounding and filling |
| Class C | ISO 7 | 352,000 particles/m³ (Static) | Preparation, filtration, and pre-filling processes for non-terminally sterilized products |
| Grade D | ISO 8 | 3,520,000 particles/m³ (Static) | Raw material weighing, packaging, general production areas |
Note: Class A/B requires unidirectional flow (laminar flow) systems with air velocity controlled at 0.36–0.54 m/s; Class C/D utilizes non-unidirectional flow (turbulent flow).
Key Control Elements
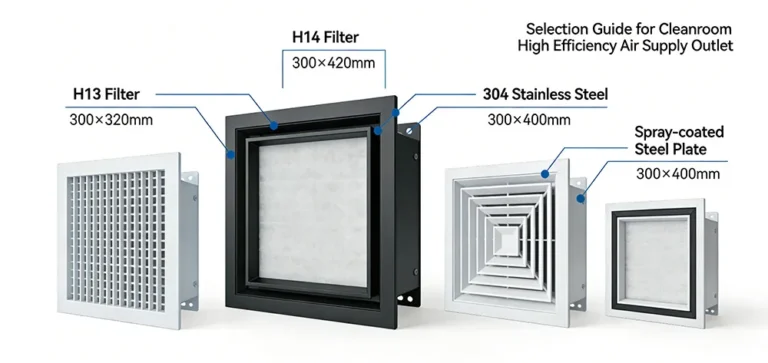
- Air Filtration: Utilizes HEPA (H13 grade) filters with ≥99.95% filtration efficiency for particles ≥0.3μm.
- Pressure Differential Control: Clean zone > Non-clean zone (≥10 Pa), with Class A > Class B > Class C > Class D forming an “air barrier.”
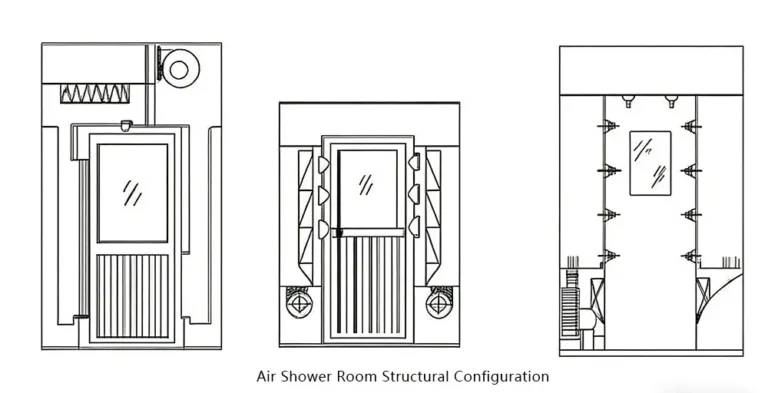
- Personnel Management: Personnel represent the primary contamination source (over 80%). Entry requires multiple procedures including gowning, air showers, and hand disinfection.
- Environmental Monitoring: Real-time monitoring of temperature, humidity, pressure differential, particle counts, airborne microorganisms, and settled microorganisms. Data must be traceable and tamper-proof.
Question 1: Why must pharmaceutical cleanrooms use HEPA filters? Can they truly filter out viruses?
Answer: HEPA (High Efficiency Particulate Air) filters serve as the “last line of defense” in cleanrooms. Their operation relies on a quadruple mechanism: interception, inertial impaction, diffusion effects, and electrostatic adsorption.
- Bacteria, mold spores (1–10μm): Captured through interception and inertial collision;
- Viruses (0.02–0.3μm): Though smaller than 0.3μm, enhanced Brownian motion increases collision probability with fibers, enabling efficient capture;
- 0.3μm particles: The most challenging to filter, serving as the benchmark for HEPA efficiency testing, with H13-grade filtration efficiency ≥99.95%.
Case Study: After upgrading to H13-grade HEPA filters, a vaccine manufacturer reduced total airborne bacteria from 150 CFU/m³ to 8 CFU/m³, achieving a 94.7% reduction.
Conclusion: While HEPA filters cannot “kill” microorganisms, they physically intercept the vast majority of pathogens carried by aerosols, serving as the technological cornerstone for meeting GMP sterility requirements.
Question 2: If HEPA filters exist, why can’t pharmaceutical cleanrooms achieve “complete sterility”?
Answer:The objective of cleanrooms is “controlled low contamination,” not “absolute sterility.” The reasons are as follows:
1.Persistent pollution sources:
- Personnel activities (breathing, skin shedding, clothing friction) continuously release particles and microorganisms;
- Equipment operation (pumps, mixers) generates friction particles;
- Material transfer (material entering/exiting pass-throughs) introduces external contamination.
2. Microorganisms possess “concealment” and “reproductive capacity”:
- Certain microorganisms (such as spores and mold) can adhere to equipment crevices, return air ducts, and sealant joints, forming biofilms;
- Filter media itself acts as a “microbial collector.” If not regularly disinfected during prolonged operation, replacing it may cause aerosol leakage.
3. Verification standards permit “ultra-low thresholds”:
- Class A zones permit ≤1 CFU/4-hour sedimentation bacteria, Class B ≤5 CFU/4 hours—not “zero,” but “acceptable risk.”
Scientific consensus: Cleanrooms are risk control systems, not “sterile vacuums.” Their value lies in reducing contamination probability to clinically acceptable levels, ensuring patient safety.
Core Benefits of Implementing Cleanrooms
For pharmaceutical companies, constructing and maintaining cleanrooms represents a three-pronged strategy encompassing compliance, quality, and business survival. The benefits are supported by clear data:
| Benefit Dimension | Specific Manifestations | Data Support |
| Improving Drug Compliance Rates | Reducing batch rejections caused by microbial contamination and particulate matter | Following cleanroom upgrades, a solid oral dosage manufacturer reduced product non-compliance rates from 3.2% to 0.4%, achieving annual savings exceeding 8 million yuan. |
| Reduce product recall risks | Avoid FDA warning letters or mandatory recalls due to contamination | 2023 FDA data indicates that 72% of drug recalls are linked to failures in production environment control. Cleanroom compliance can reduce such risks by over 80%. |
| Ensure GMP Compliance | Pass FDA, EMA, and NMPA audits to avoid production halts and corrective actions | After completing cleanroom IQ/OQ/PQ validation, a pharmaceutical company reduced FDA 483 observations from 12 to 2 and shortened the certification cycle by 40%. |
| Enhance market competitiveness | Meet international clients’ requirements for aseptic production qualifications and access European and American markets | Products from EU GMP-certified enterprises command export prices averaging 15–25% higher and secure long-term orders more readily. |
| Supporting Innovative Therapies | Providing a stable production environment for highly sensitive products such as cell and gene therapies, and microbiome therapies | Due to the sensitivity of live bacteria in microbiome therapies, fluctuations in cleanroom environments can cause microbial imbalance, leading to a decrease in pass rate exceeding 50%. |
Core Conclusion: Cleanrooms are not cost centers but quality investments, yielding returns across three dimensions: compliance, patient safety, and business sustainability.
Its core characteristics and composition can be summarized in three points:
- Core Control Objectives : First, control particulate matter to prevent contamination of pharmaceuticals and maintain purity.Second, control microorganisms to prevent contamination and degradation of pharmaceuticals, particularly for sterile products such as injectables and ophthalmic preparations.
- Critical Facility Systems : Includes air purification systems (primary + medium + high-efficiency three-stage filtration), temperature/humidity and differential pressure control systems, sealed enclosure structures (color-coated steel panel walls, stainless steel flooring, and other easy-to-clean materials), along with auxiliary facilities such as air showers, pass-through chambers, and clean benches to achieve clean control and isolation of personnel and material flows.
- Cleanliness Grade Classification : The pharmaceutical industry adheres to GMP standards, categorizing cleanrooms into four grades—A, B, C, and D—corresponding to different levels under the international ISO 14644-1 standard. Grade A represents the highest cleanliness level, suitable for high-risk operations such as aseptic filling and packaging. Grade D denotes the lowest cleanliness level, primarily used for oral dosage production and pharmaceutical outer packaging processes.
In simple terms, pharmaceutical cleanrooms serve as the “sterile safety shield” for drugs, forming the core infrastructure for compliant manufacturing in pharmaceutical companies.