Products
Featured products
The isolator adopts the ISOLATOR safety level with environmental control meeting Class A standards. It integrates seamlessly into Class D or CNC environment laboratories, eliminating the need for complex B-level zone gowning procedures. Operators are completely isolated from test samples, eliminating cross-contamination risks and fundamentally resolving false positives to ensure drug release. It also significantly reduces environmental operating costs.Integrated with a sterile pass-through window and hydrogen peroxide sterilization/decontamination technology, it enables efficient, rapid sterilization of samples prior to testing, further mitigating risks.The equipment accommodates diverse batch sizes and process requirements.
Features:
- Modular design, configuration, loading capacity, and structural combinations can be flexibly selected based on customer testing batch sizes.
- Features a seated operation mode to reduce work fatigue and enhance operational accuracy.
- Supports handling of toxic materials with a system enabling safe filter replacement.
- Class 100 Vertical Laminar Flow
- Adjustable positive/negative pressure
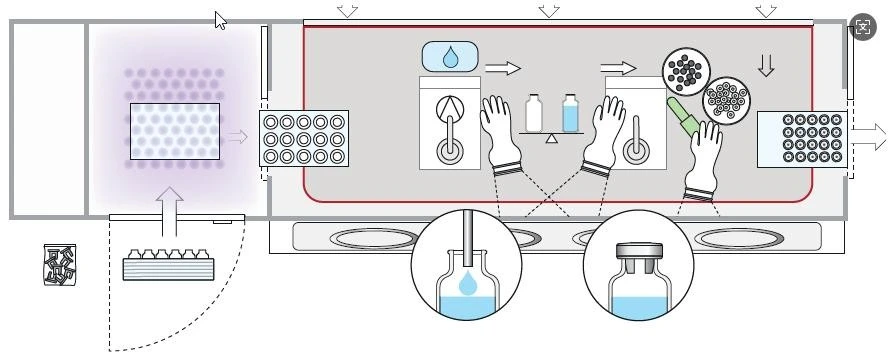
Isolators are mainly used in the pharmaceutical industry to provide a physical barrier to protect the sterile environment and personnel safety. It consists of an operating cabin and a transfer cabin, with a positive pressure fully enclosed design. The airflow pattern is similar to that of a biological safety cabinet, but the pressure is opposite. It supports processes such as filling, weighing, and batching, and can be customized in size and equipped with an optional VHP sterilization system.
Aseptic isolators are used for sterile production and testing, meet Class A clean standards, use stainless steel/tempered glass hard cabins, and have laminar or turbulent flow designs. The closed isolator completely isolates personnel and processes to prevent contamination, and is suitable for sterile inspections, microbial testing, etc. Operations are performed using gloves, and materials enter and exit the transfer cabin.
Sterility Testing Isolator (Rigid Chamber) Key Technical Parameters:
| Model | HSM-lL |
| Power Supply | AC220V+22V/50Hz |
| Power Consumption | 3KW |
| Dimensions | 2530*1250*2290mm (L*W*H) |
| Noise Level | ≤70dB(A) |
| Pressure Differential | 0~80Pa (adjustable) |
| Cleanliness Level | Class A (static) |
| Sterilization Rate SAL | 6Log |
Contact us at boben Modular Cleanroom Manufacturer. We will provide a detailed, customized sterile isolator solution and technical quotation tailored to your specific process requirements (such as filling speed, material characteristics, required VHP cycle time, etc.), aligning with your budget and standards.
What are the application scenarios of sterile isolators?
Vaccine aseptic filling
- Requirement:Ensure the sterility of the mRNA vaccine filling process in compliance with FDA/EMA regulations.
- Solution:Fully automated isolator with integrated VHP sterilization (log6 microbial kill). Equipped with Class A laminar flow (ISO Class 5) and online particle monitoring system.
- Result:Achieved zero-contamination batch production and passed FDA on-site audit.Filling efficiency increased by 30% (compared to traditional RABS system).
Cell therapy product preparation
- Requirement:Sterility and cross-contamination prevention and control of CAR-T cell processing.
- Solution:Small biosafety isolator (BSL-2 level) with integrated CO₂ culture module.Single-person mobile design saves clean room space.
- Result:The contamination rate of cell products has been reduced from 5% to 0.2%.50 clinical-grade cell preparations have been successfully completed.
Choose Boben, choose expertise and trust.
As a globally renowned modular cleanroom manufacturer, we specialize in delivering high-standard clean solutions. Our products are exported to five continents and successfully deployed by numerous internationally renowned pharmaceutical companies and research institutions. We provide not just equipment, but a comprehensive sterile process assurance system.
By choosing us, you gain:
Act Now:Customize Your Rapid Clean Space!
Don’t let lengthy lead times slow down your production schedule.Contact our cleanroom experts for a free design consultation and customized quote to kickstart your fast-track production journey.










